Introduction
Intravenous (IV) daratumumab has become a standard in the treatment of MM and AL amyloidosis largely due to its significant clinical benefit. Due to the high risk of infusion-related reactions (IRRs), it is associated with prolonged infusion times. These lengthy administrations can limit clinic capacity, require split dose infusions, increase chair time, decrease patient satisfaction, and create access barriers in the era of COVID-19. Recently, a fixed-dose subcutaneous (SC) formulation of daratumumab was approved for the treatment of MM, and the safety-run in results (N=28 patients) from a phase III, randomized trial in AL amyloidosis using the SC formulation were published. The SC formulation has the potential to mitigate some of the setbacks from IV formulation and help improve efficiency in the era of COVID-19, where most clinics are faced with limiting and spreading out volume due to space constraints and social distancing requirements. We present real world evidence from a large academic center's experience with adoption of SC formulation to help overcome current challenges.
Methods
We prospectively reviewed all patients being treated with IV daratumumab for MM and/or AL amyloidosis at Boston Medical Center Health System (a 500+ bed integrated delivery network) that would be candidates for the SC formulation. A protocol was designed to switch patients that were currently on IV daratumumab as well as patients newly initiating daratumumab to the SC formulation. Patients deemed eligible were switched to the SC formulation under pharmacy benefit (pending insurance authorization by a pharmacy liaison) at their next scheduled infusion visit. Rationale for switching patients to pharmacy benefit was to support home administration during future surge from COVID-19, using our specialty pharmacy travel RN program. Patients that were naïve to daratumumab, started their first dose as a SC injection. Patients were monitored for 30 minutes following the first SC dose and were pre-medicated with oral acetaminophen, dexamethasone, and diphenhydramine. Patient and nursing satisfaction were assessed after switching to SC daratumumab, using internally developed surveys. Using wholesale acquisition cost, cost analysis was performed between the two formulations. Infusion chair time, improvement in clinic efficiency (using our standard infusion time of 90 minutes for IV daratumumab infusions after the 2nd dose), and safety were assessed and compared.
Results
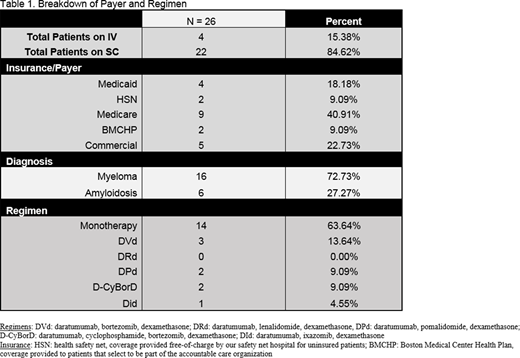
A total of 26 patients were treated with daratumumab for MM and AL amyloidosis from June 1, 2020 to August 1, 2020. 85% (22/26) of patients were administered the SC formulation. Of these 22 patients, 68% of patients were switched from the IV formulation (15/22) and 32% (7/22) were naïve to daratumumab. The majority (14/22) of SC administrations were patients receiving daratumumab monotherapy for relapsed MM. A breakdown of patient regimens and insurance type is seen in table 1. IRRs were reported in 0 patients starting [or transitioning to] SC daratumumab. Injection site reactions were not reported in any patient. One patient had facial and neck swelling 2 days after administration of SC daratumumab (with no other symptoms) but resolved within 24 hours of an additional dose of dexamethasone and did not recur upon re-challenge of SC daratumumab. Severe neutropenia (ANC less than 500) was reported in 9% of patients (2/22), both patients in the group naïve to daratumumab. Febrile neutropenia was not seen. One patient was being treated with concomitant pomalidomide and the second was treated with lenalidomide and had a baseline ANC of 500 prior to initiation of therapy. Adoption of SC daratumumab led to elimination of 133 hours of chair time and nursing time, or 1260 hours annualized for the year. Cost savings with the elimination of nursing time translates to approximately $100,000 to the institution and approximately $230,000 to the payer. The results compiled from patient and nursing satisfaction surveys are being analyzed and will be presented.
Conclusion
We report a successful conversion and adoption of SC daratumumab at our ambulatory hematology/oncology clinic. Insurance authorization does not appear to limit the adoption of this therapy in clinic irrespective of diagnosis or regimen used. Furthermore, the reduction in chair time and patient convenience was largely beneficial in light of COVID-19 to minimize patient exposure in clinic.
Hughes:Rigel: Other: advisory board; Abbvie: Speakers Bureau; Amgen: Speakers Bureau; Karyopharm: Speakers Bureau. Blevins:Epizyme: Other: Focus Group. Sarosiek:Spectrum: Research Funding. Sloan:Abbvie: Consultancy; Stemline: Consultancy. Sanchorawala:Celgene: Research Funding; Takeda: Research Funding; Caleum: Other: advisory board; Proclara: Other: advisory board; Regeneron: Other: advisory board; Abbvie: Other: advisory board; Janssen: Research Funding; UpToDate: Patents & Royalties; Oncopeptide: Research Funding; Prothena: Research Funding; Caelum: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal